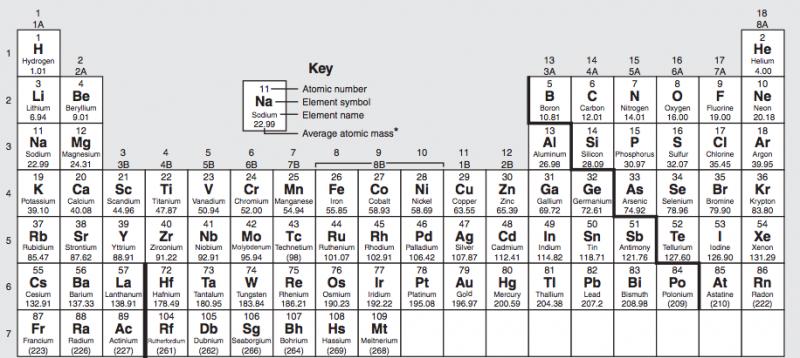
The element with the highest electronegativity.
The element with the highest ionization energy.
The element with the smallest atomic size.
The element with the lowest electronegativity in period 4.
The element with the highest metallic character.
The element with the largest atomic size in period 6.
The element with the lowest electronegativity of the halides.
The element with the highest electronegativity of group 2.
The element with the highest ionization energy of group 5.
The element with the largest atomic size of group 3.
The element with the greatest number of orbitals in group 8.
The element with the smallest nuclear charge in period 3.
The element with the largest nuclear charge in group 4.
The element with the smallest number of orbitals in group 1.
The element that forms -3 ions in period 4.
The elements that forms +2 ions in period 4.
The element that forms +1 ions in period 5.
The element that forms -2 ions in period 4.